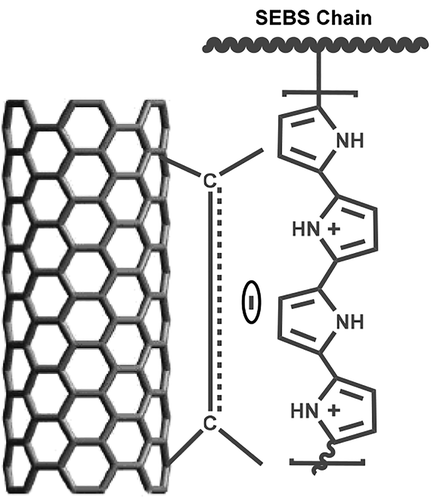
Polypyrrole grafted polystyrene-b-poly(ethylene-ran-butylene)-b-polystyrene (SEBS-g-PPy)/multiwall carbon nanotubes (MWCNTs) conductive nanocomposites were fabricated using two different approaches. The approach of system-I involved primarily the grafting of PPy on SEBS and its subsequent composites with nanotubes. In system-II in situ polymerization/grafting of PPy on SEBS was carried out along with MWCNTs yielding nanomaterials. Presynthesized SEBS-g-PPy and nanocomposites were characterized by Fourier transform infrared spectroscopy, NMR, field emission scanning electron microscopy, transmission electron microscopy, and electrical, mechanical, and thermal properties. The π–π stacking interactions between PPy of SEBS-g-PPy and MWCNTs rendered ample dispersion of the nanotubes in system-II relative to system-I. The electrical conductivity and tensile data showed improvement in these properties of nanocomposites and that system-II nanocomposites can sustain higher stresses, is stiffer, and can absorb more energy before breaking. Thermal stability of both the systems was improved relative to the matrices, and decomposition temperatures were found to increase from 437 to 568 °C. Relative improvement in electrical, thermal and tensile properties were observed for system-II nanocomposites rather than for system-I nanocomposites.