author:REGEN-αGEEK
page views:1293

2024-12-11
share
On December 10, 2024, the "Recombinant Collagen Protein Liquid Dressing," declared by Guangdong Ruicheng Medical Technology Co., Ltd., an ecological enterprise under REGEN-αGEEK, was officially approved for a Class II Medical Device Registration Certificate (Certificate No.: Yue Xie Zhu Zhun 20242141656) issued by the Guangdong Medical Products Administration. This marks Guangdong Province's first* liquid recombinant collagen protein dressing to receive a Class II Medical Device Registration Certificate, achieving a breakthrough of "zero" for Class II medical device registrations of recombinant collagen protein products in Guangdong Province (*Source: Guangdong Medical Products Administration data query database).
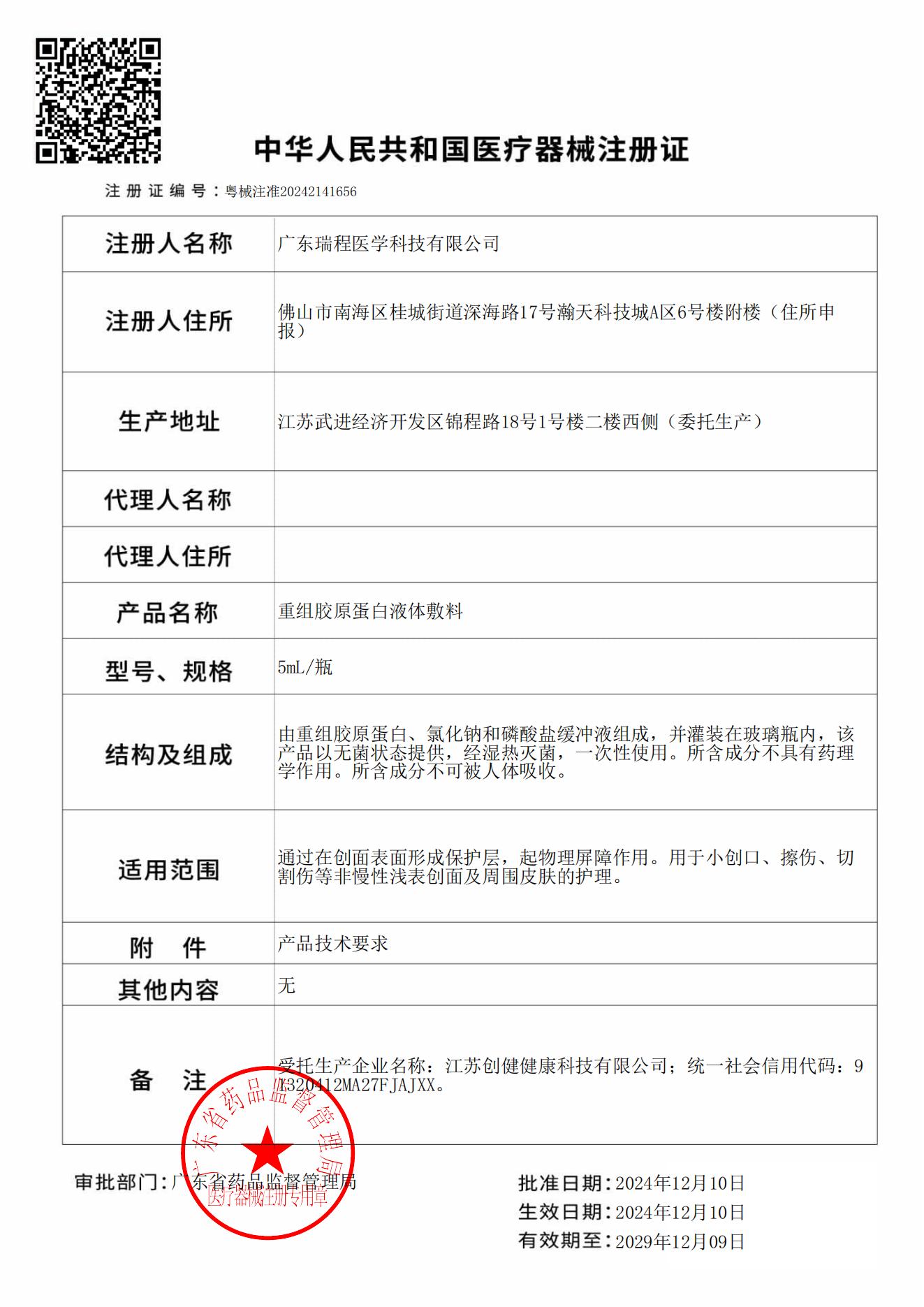
This "Recombinant Collagen Protein Liquid Dressing" product primarily functions by forming a protective layer on the wound surface that acts as a physical barrier. It is intended for the care of non-chronic superficial wounds—such as minor cuts, abrasions, and incisions—and the surrounding skin. The approval of the National Class II Medical Device Registration Certificate represents full recognition by China's national regulatory authority of the product's quality standards.
Hot

2025-05-06

2025-03-20

2025-01-09

2024-12-11

2024-11-02

2024-10-26

2024-07-02

2023-10-31
Related tags






