author:REGEN-αGEEK
page views:1646

2025-05-06
share
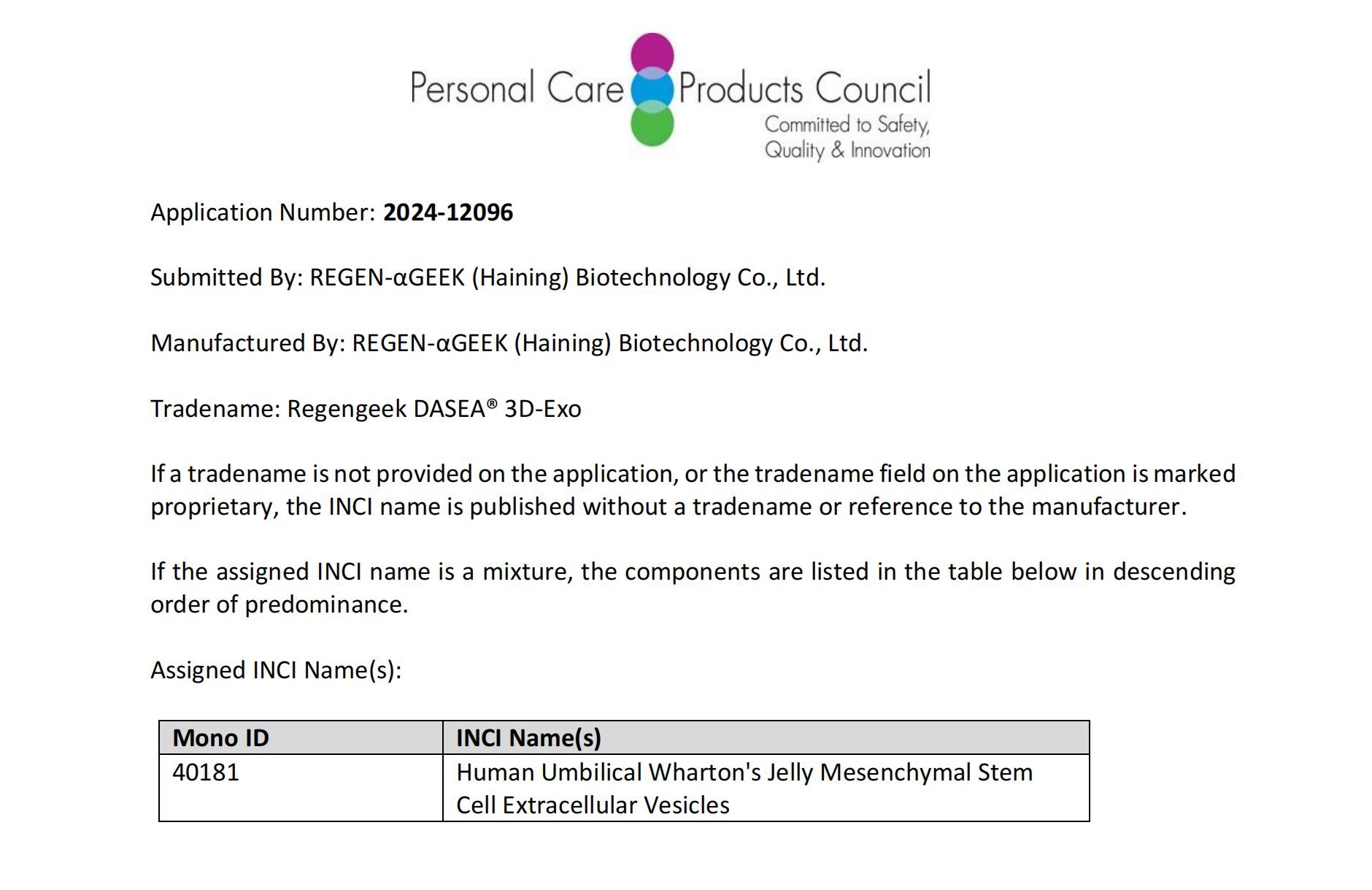
On May 1st, human umbilical cord Wharton's Jelly mesenchymal stem cell extracellular vesicles (English Name: Human Umbilical Wharton's Jelly Mesenchymal Stem Cell Extracellular Vesicles INCI, Number: 40181), prepared using REGEN-αGEEK DASEA®'s 3D biomanufacturing platform, were formally approved for inclusion in the International Nomenclature of Cosmetic Ingredients (INCI) Directory. This achievement follows the product's successful dual Drug Master File (DMF) filings with both the Center for Biologics Evaluation and Research (CBER) and the Center for Drug Evaluation and Research (CDER), agencies under the US Food and Drug Administration (FDA). This renewed recognition by an international authority fully reflects the safety, stability, and efficacy of the exosomes prepared via DASEA®'s 3D biomanufacturing platform.
Hot

2025-05-06

2025-03-20

2025-01-09

2024-12-11

2024-11-02

2024-10-26

2024-07-02

2023-10-31
Related tags






