

The human umbilical cord mesenchymal stem cells produced by REGEN-αGEEK have been officially certified by the National Institutes for Food and Drug Control (NIFDC) of China, with 6 NIFDC testing reports obtained.

REGEN-αGEEK has obtained 18 U.S. DMF filings and 2 CE filings for its product portfolio. The production and quality control of these products adhere to international standards, meeting customer needs at all stages and supporting compliant drug registration.
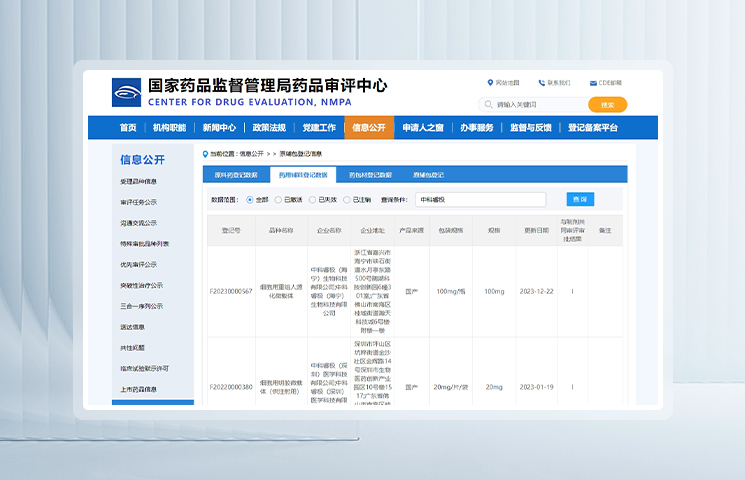
Two microcarriers from REGEN-αGEEK have successfully obtained the pharmaceutical excipient filing approval from the CDE of the NMPA (Registration Numbers: F2023000567, F20220000380).

NMPA Class I device No. : Yue Chan 20230036, 20240142, 20240151, 20250019 & 20250079; Completed the production registration of Class I medical devices for in vitro diagnostic reagents, ensuring that the products comply with regulatory requirements.

ISO 13485:2016 medical devices quality management system
ISO 9001:2015 quality management system
ISO 14001:2015 environmental management system
ISO 45001:2018 occupational health and safety management system
By adhering to standardized and regulated management processes, we enhance the quality level of our products and services to meet customer needs and expectations.

Applied 118 PCs and 83 granted patents (as of May 2025)

Organize & participate in 8 group standards






