
Building the core “new productive capacity” in the CGT
Bottlenecking Problems of 2D
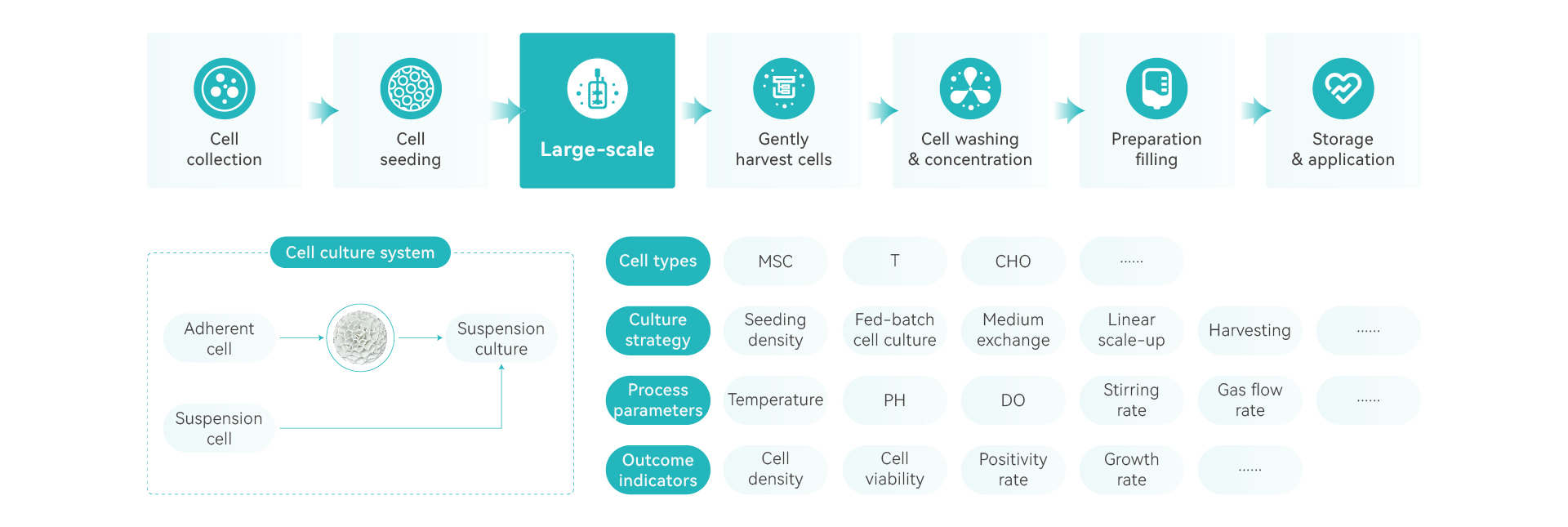
Advantages of DASEA® Bio-manufacturing Platform
subjectively ambiguous


digitalization of whole process and consumables
high labor and consumable costs


automatic and programable process control
insufficient use of space


3D culture, large single-batch production yield
open space operation


enclosed system, less contamination
repeated enzyme digestion


better cell activity with biomimetic microcarriers
Expected cell harvest
Type of cultivation
Facility
Time
Labor
Reagent & consumables (thousand yuan)
Total cost (thousand yuan)
1*1010cells
2D
200㎡
1200h
10 pers
19.53
28.32
DASEA® 3D
100㎡
120h
1 pers
9.60
13.30

90
%Facility

90
%Labour

80
%Time

52
%Reagents & consumables

62
%Total cost
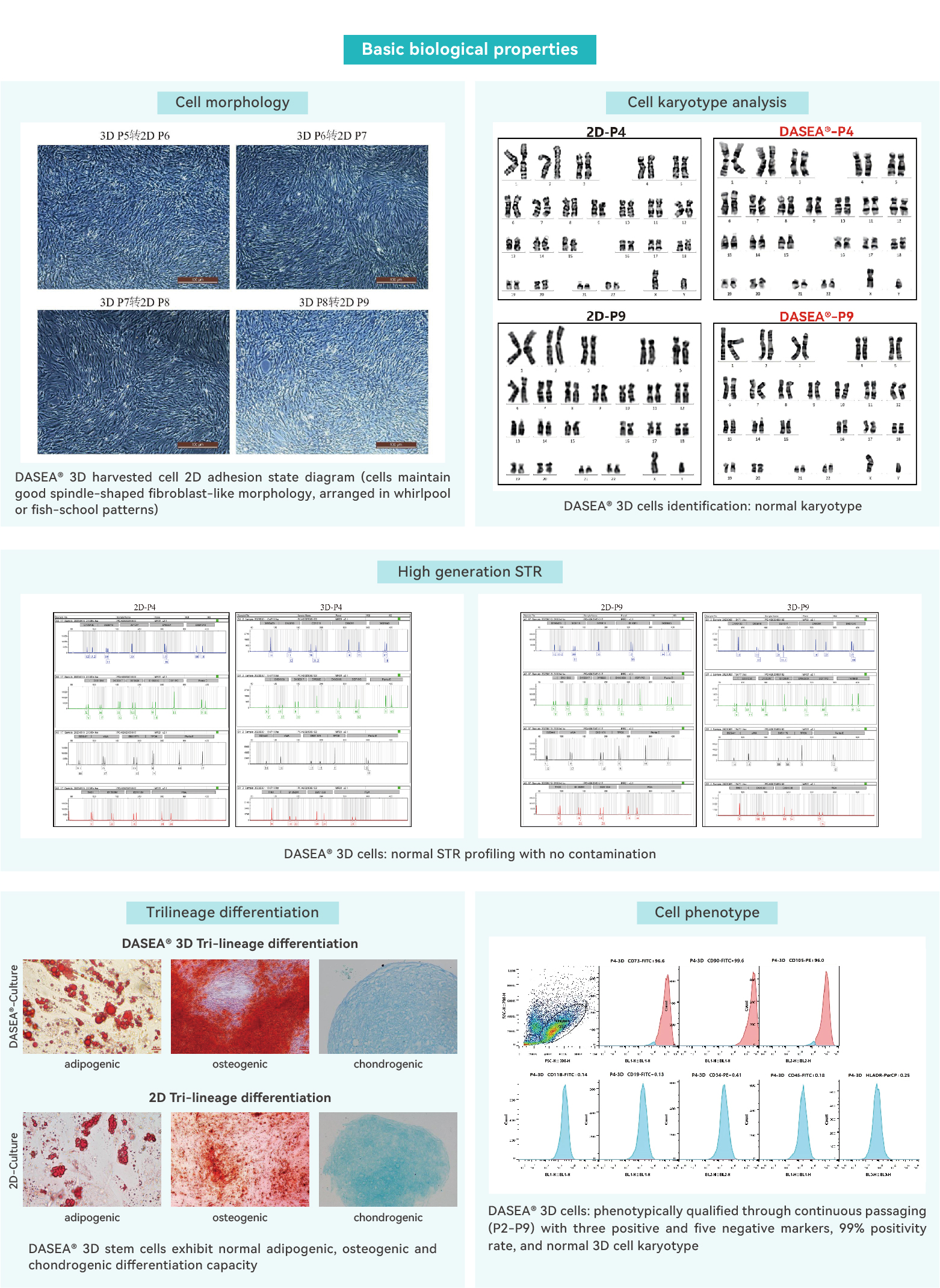
Cell morphology / Viability / Cell count
Surface marker analysis of hUMSCs
Human STR profiling identification
Induced in vitro differentiation capacity test (Tri-lineage differentiation)
Chromosomal karyotyping
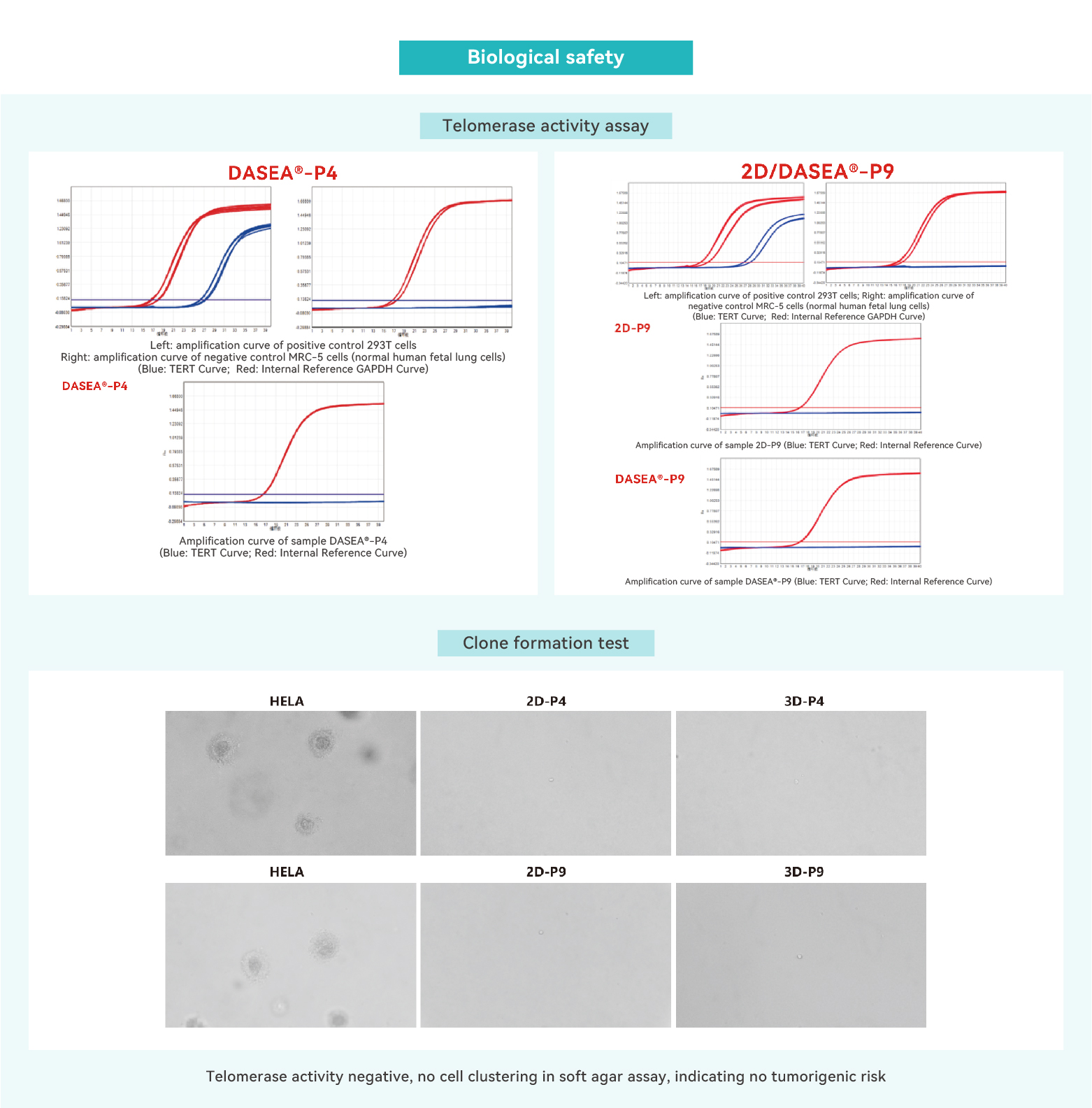
Telomerase activity assay
Soft agar colony formation assay
Nude mouse inoculation test
Bacterial/Fungal detection
Mycoplasma detection
Adventitious virus testing (Human / Animal-derived)
Endotoxin testing
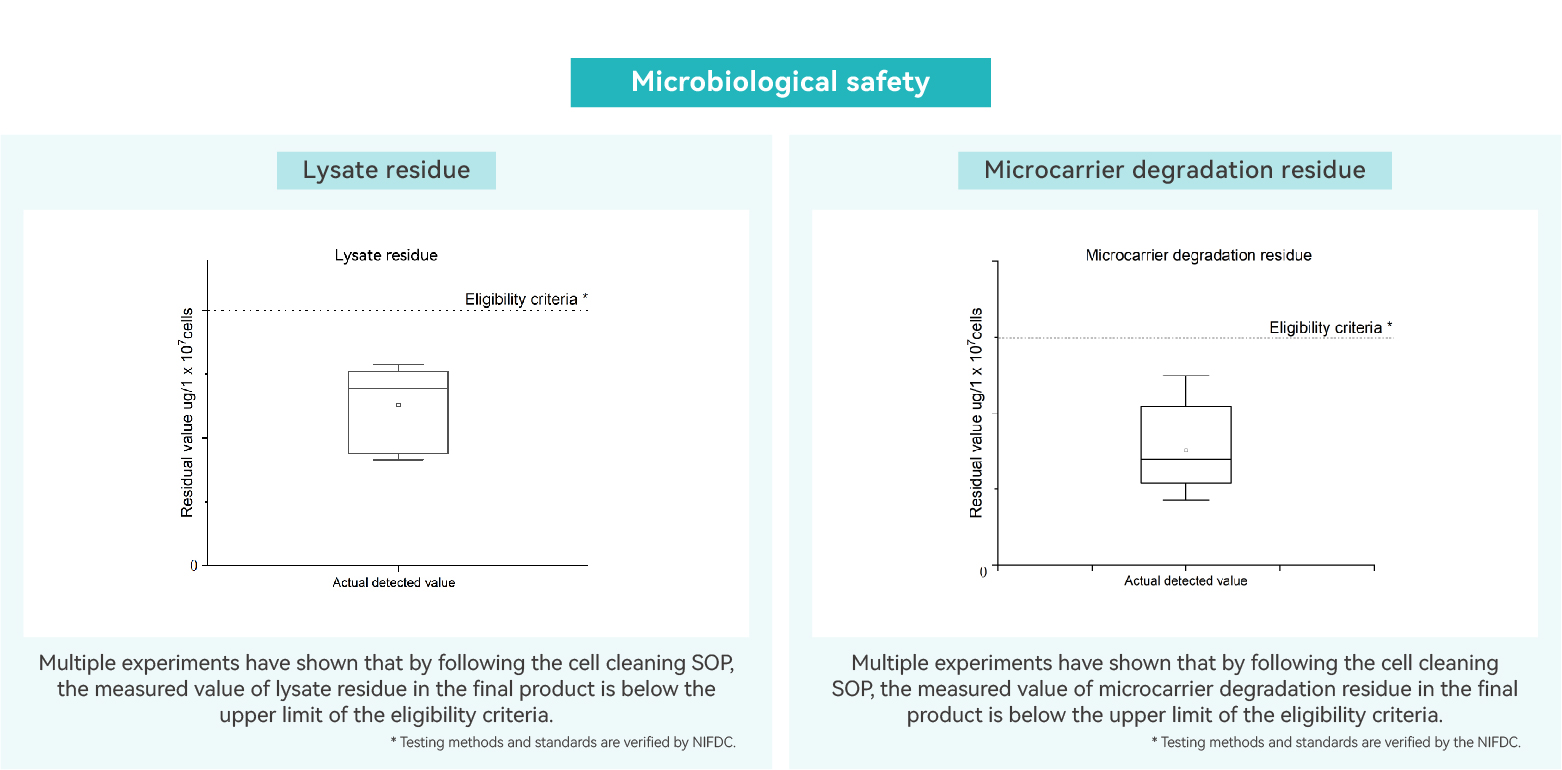
Lysate residue
Microcarrier lysate residue testing
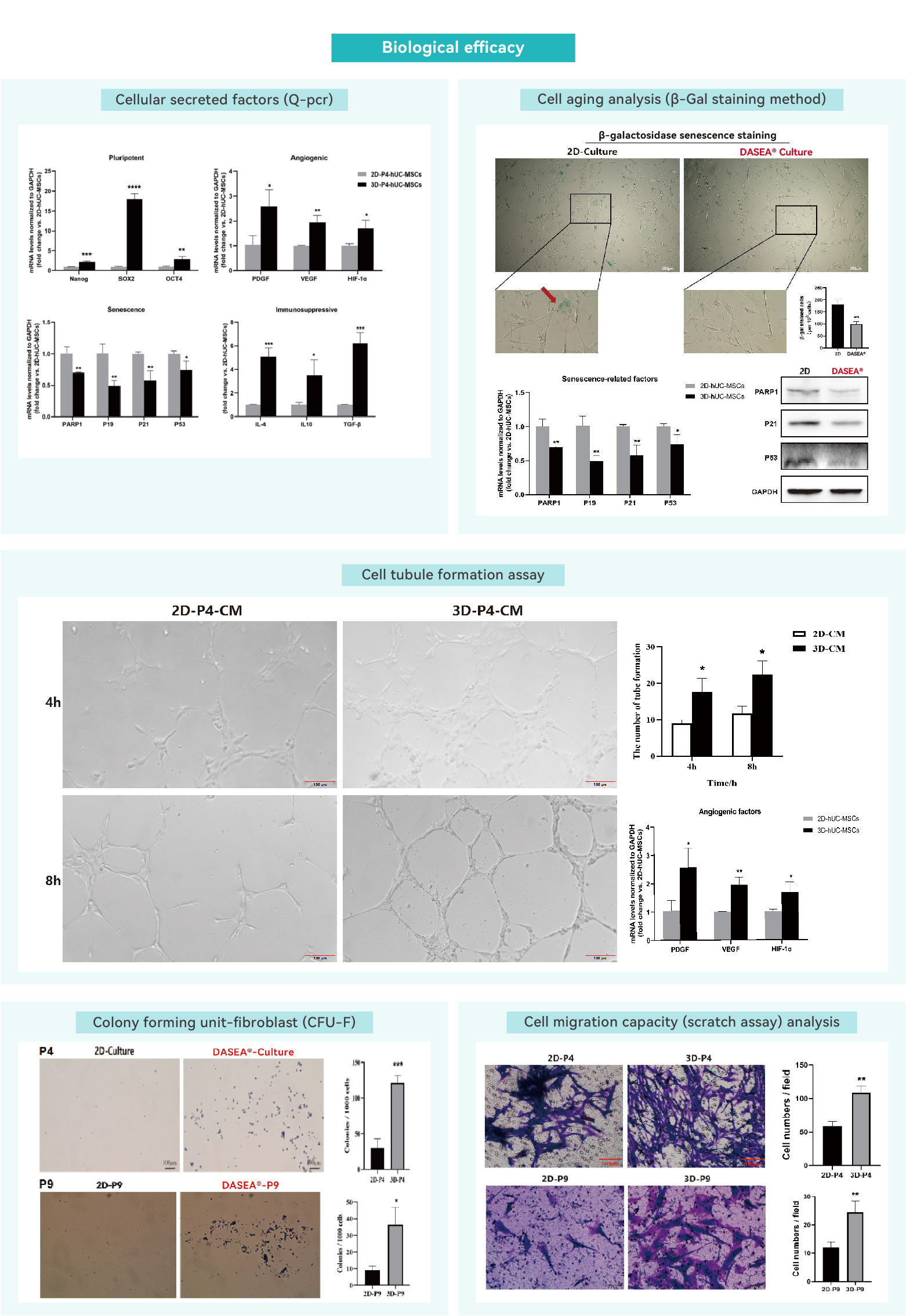
Cell secretory factors
Cellular senescence assay
Angiogenesis assay
Fibroblast colony-forming units (CFU-F)
Cell migration
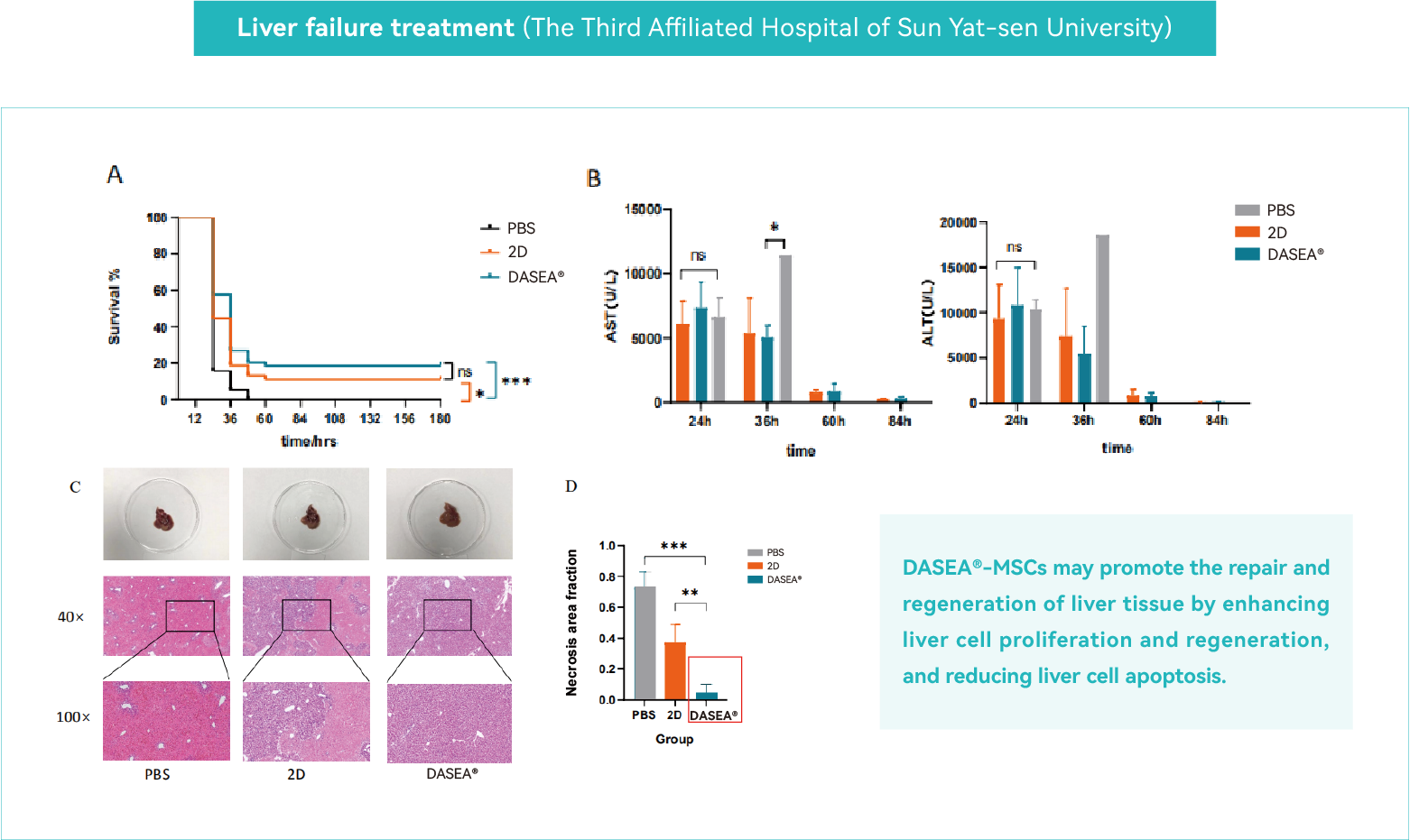
Liver Failure Treatment
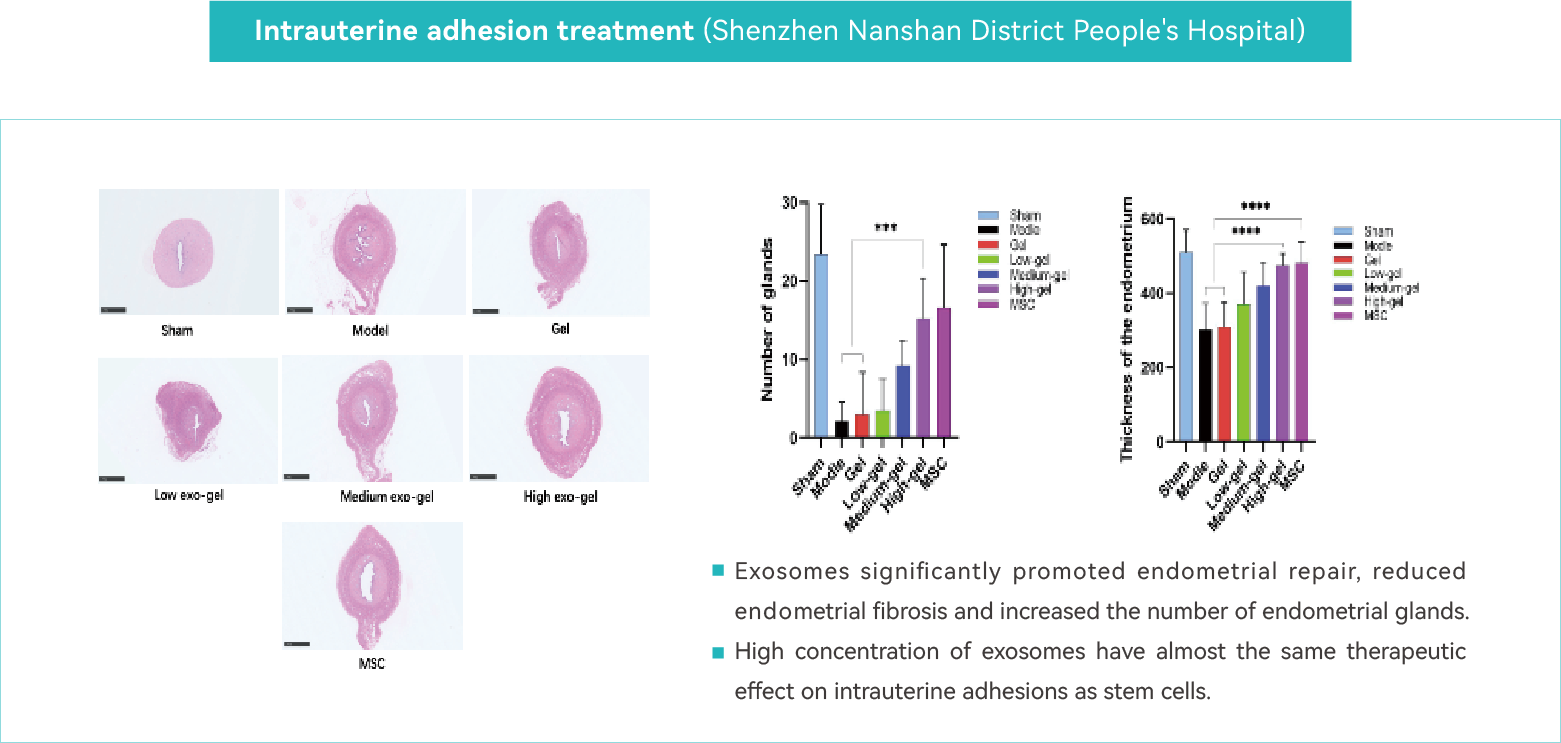
Intrauterine Adhesion Treatment
The statistical data as of Nov 4, 2024
Indications
Mechanism study
Production process
Quality inspection
Pre-clinical study
Investigator-Initiated Trial
Intrauterine adhesions
Intervertebral disc degeneration
Refractory wounds
Acute liver failure
Chronic kidney disease
Tendon injur






